Didier Rigollet, Amélie Havard and Daniel Dron are working at the R&D department Analytical Innovative Technologies of the Industrial Research Centre at Oril Industrie, in Bolbec, France (Servier group). The use of HPTLC in Oril started even earlier than the foundation of Chromacim, including trainings at CAMAG organised by Pierre Bernard-Savary, where Daniel Dron participated already more than 20 years ago. The R&D team is specialized in purification processes of intermediates and active pharmaceutical ingredients (APIs) for toxicological, galenical or clinical studies. Part of their work is devoted to the isolation of impurities and production of APIs or impurity reference batches. In 2018, the team launched their preparative chromatography service InnoPrepTM, dedicated to small and large-scale purifications. Intermediates, APIs and impurities are characterized by MS and NMR. Quantitative analysis by NMR is also performed on these molecules.
Introduction
Servier, an independent international pharmaceutical group, is committed to therapeutic progress for the benefit of patients.
Their goal is to speed up the development of new molecules in order to bring a new molecular entity to market every 3 years, particularly in the field of oncology. Preparative chromatography is, therefore, a method of choice in R&D to provide pure products for the first pharmacological, toxicological and clinical studies in a very short time. This technique also makes it possible to isolate impurities present at low levels in active ingredients whose complex structure does not allow rapid synthesis and to provide a batch within the time limits set for toxicological studies.
About 75% of the purifications at Oril Industrie are done with silica gel. The necessary conditions for an efficient purification are determined using TLC. Then, the purification progress by preparative column chromatography is checked by HPTLC. Twenty fractions can be analyzed within one hour. TLC/HPTLC is the method of choice due to its simplicity, rapidness and the successful scale up from TLC to preparative separations. The HPTLC-MS carried out beforehand allows targeting the molecule sought in often very complex mixtures.
Sample preparation
Crude product (0.05 g) is dissolved in 5.0 mL of ethyl acetate.
Chromatogram layer
For method development (optimization of purification conditions) TLC plates silica gel 60 F254 (Merck), 20 x 5 cm are used, while quantification and coupling to mass spectrometry is done on HPTLC plates silica gel 60 F254 s (Merck), 20 x 10 cm.
Sample application
Samples are applied as bands with the Automatic TLC Sampler (ATS 4), two tracks for TLC and up to 20 tracks for HPTLC, band length 8.0 mm, sample volumes of 1.0 –15.0 μL.
Chromatography
Plates are developed in the Twin Trough Chamber 20 x 20 cm (TLC) or 20 x 10 cm (HPTLC) with chamber saturation (with filter paper) for 20 min with different developing solvents to the separation distance of 100 mm (from the lower edge) for TLC and 50 mm for HPTLC, followed by drying in a stream of cold air for 5 min.
Documentation
Images of the plates are captured with the TLC Visualizer in UV 254 nm and white light.
Mass spectrometry
Zones are eluted with the TLC-MS Interface (oval elution head) at a flow rate of 0.2 mL/min with methanol – water 1:1 (V/V) into a Q-TOF-MS (Xevo® G2-S QTof, Waters), operating in positive ionization mode (m/z 50 –1200).
Results and discussion
The objective of this study was to isolate a sufficient quantity of an impurity present at a content of 0.35% in a batch of an intermediate in a product under development. The aim was to confirm its structure and to carry out toxicological tests. The nature of this impurity was determined beforehand by LC-MS. Its structure being complex, a synthesis would be too time-consuming. The conditions used in RP-HPLC are too complex for direct transfer to preparative chromatography (expensive stationary phase). The mobile phase used was water + 0.1% methane sulfonic acid and acetonitrile +0.1% methane sulfonic acid is too complexed for the isolation after preparative chromatography.
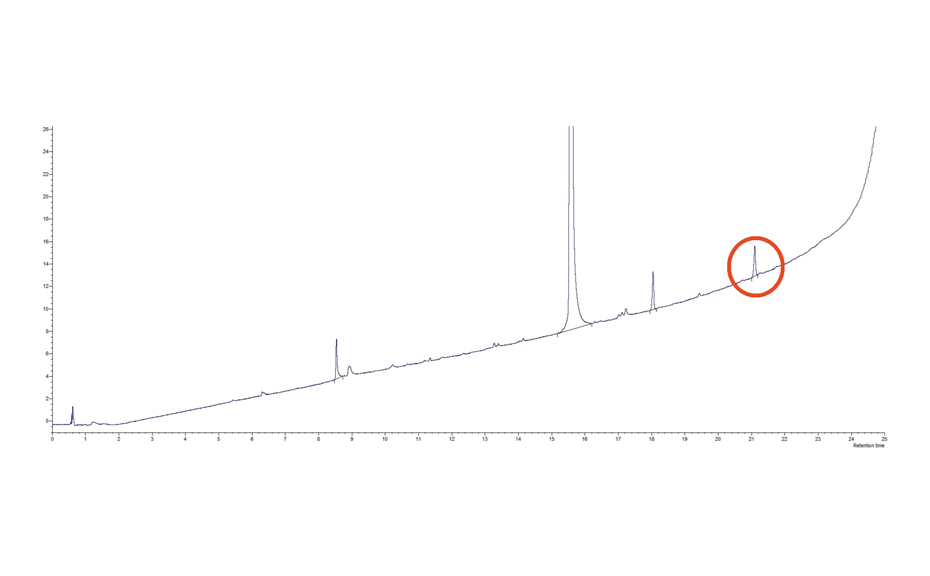
Figure 1: RP-LC-UV Chromatogram of the crude product
TLC was selected for method development to separate the major impurity from the other compounds with a reasonable RF value allowing an efficient purification.
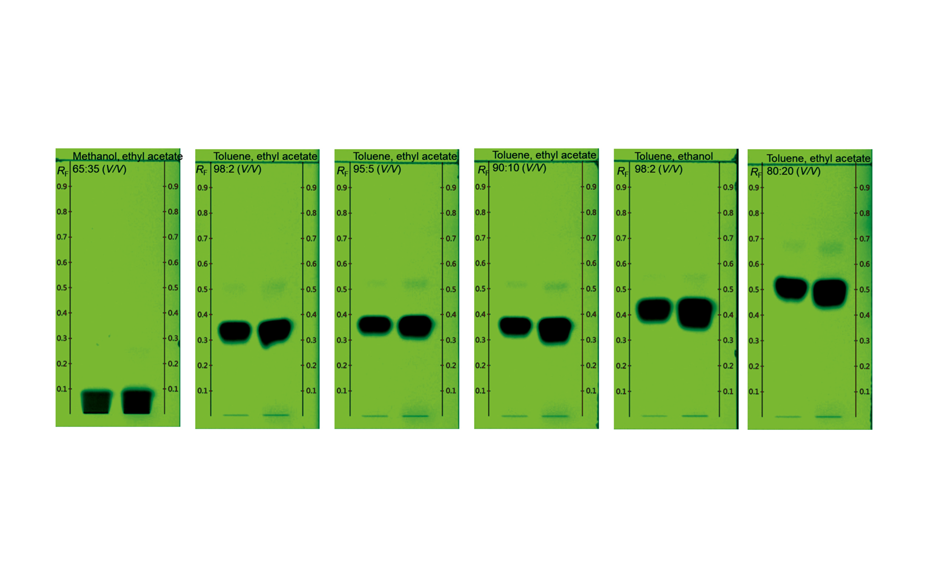
Figure 2: TLC chromatograms of the crude product in UV 254 nm obtained with different developing solvents
Mass spectra were recorded to characterize the different compounds (main substance at RF 0.25 and impurity at RF 0.38).
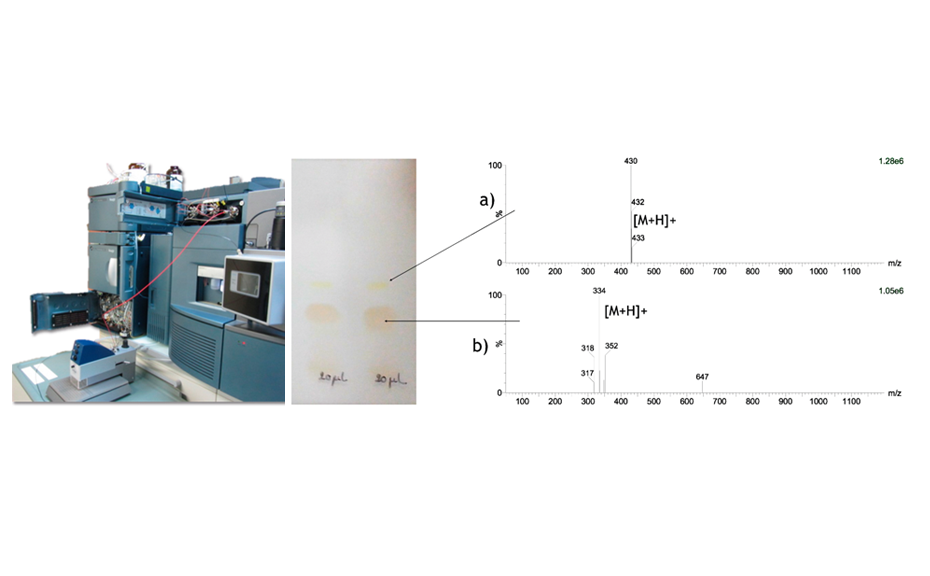
Figure 3: Instruments used for TLC-MS (left); TLC chromatogram of the main substance and the impurity in white light (middle); mass spectra of the selected zones (a: impurity, b: main substance) measured in positive ionization mode (right)
The purification of the crude product (two injections of 90g dissolved in toluene) on a 20-cm column (packed at 40 bars with 6 kg silica gel 60, 15–40 μm, Merck) at a flow rate of 2.0 L/min with toluene – ethyl acetate 95:5 (V/V), was monitored online with UV 290 nm detection and in parallel offline by HPTLC.
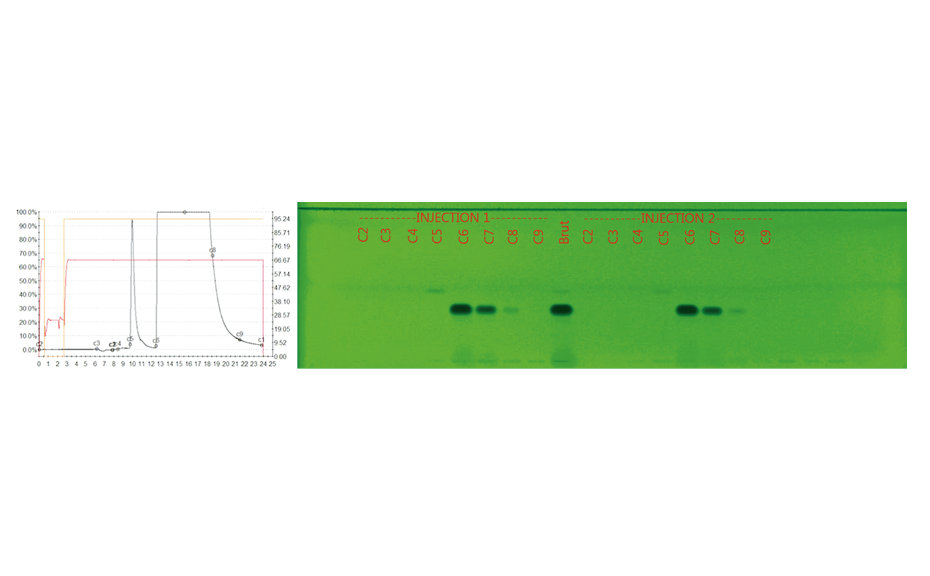
Figure 4: Online monitoring of the purification process by LC-UV (290 nm, left) versus offline by HPTLC-UV (HPTLC chromatogram of the individual fractions at 254 nm, right; C2-C9 are fractions collected during purification and C5 corresponds to the impurity)
The different fractions of the target impurity were collected, and the combined fractions were analyzed by NMR. 670 mg of impurity was obtained (yield: 0.35%) purity > 99%. The quantity obtained after purification on column was also consistent with the estimated content in analytical HPLC. For Oril, the use of HPTLC has a big positive impact on the production costs, with a benefit of thousands of Euros per year. This is due to the use of HPTLC for various optimizations of the manufacturing process and of raw materials external supply.
Further information is available on request from the authors.
Contact: Amélie Havard, Daniel Dron, Oril Industrie (Servier), Industrial Research Centre, Department of Analytical Innovative Technologies R&D, 13 rue Auguste Desgenétais, CS 60125, 76210 Bolbec, France, amelie.havard@servier.com, daniel.dron@servier.com