Standardization & SOP for HPTLC
The great advantage of TLC is its flexibility. Understanding the effects of each parameter on the outcome of the final chromatogram allows adjustments to the methodology in order to obtain the desired result. However, that degree of freedom can become a problem for reproducing a method, especially if not all parameters were documented and adhered to.
For obtaining predictable and reproducible HPTLC results, it is essential to establish a standardized methodology in the form of a standard operation procedure (SOP).
The United States Pharmacopoeia (USP-NF 2015) and the European Pharmacopoeia (Ph. Eur. 2017) published general chapters on HPTLC, which are the basis for HPTLC methods of identification as part of monographs on herbal materials. All parameters are based on an SOP adopted by the HPTLC Association in 2012.
HPTLC parameters compliant with USP and Ph. Eur.
Plate material:
- HPTLC glass plates, 20 x 10 cm, Silica gel 60 F254 (2–10 μm, average 5 μm).
Sample application:
- 8.0 mm bands, 8.0 mm from lower edge, 20.0 mm from left and right edges. The minimum distance between tracks is 11.4 mm (center to center);
- Maximum 15 tracks per plate;
- Track 1 is used for SST;
- Volumes of less than 2.0 μL and more than 20.0 μL should be avoided. Optimum application volumes are 2.0–10.0 μL.
Developing chamber and development:
- Twin Trough chamber (20 x 10 cm);
- Use 10.0 mL of developing solvent in the front trough (equivalent to a solvent level of 5 mm) and 25.0 mL for saturation in the rear trough fitted with a saturation pad (filter paper of defined thickness);
- Developing distance 7.0 cm from the lower edge of the plate or 6.2 cm from the application position;
- Prior to development, condition the plate at a relative humidity of 33% using a saturated solution of magnesium chloride for 10 minutes;
- With the lid closed, saturate the chamber for 20 minutes (with saturation pad). For development, introduce the plate in a vertical position into the front trough. The silica gel faces the saturation pad;
- When the developing solvent achieves 7.0 cm, remove the plate from the tank and dry in a vertical position with a stream of cold air for 5 min.
Derivatization:
- Automatic spraying or dipping/immersion whenever possible.
Documentation: record digital images in
- Short-wave UV light (254 nm), and white light before application as “clean plate image” for image correction;
- Short-wave UV light (254 nm), long-wave UV light (366 nm), and white light after development;
- Long-wave UV light (366 nm) and white light after derivatization.
Converting TLC/HPTLC methods into standardized HPTLC methods
As the general pharmacopoeial chapters on HPTLC were only recently published, most of the literature methods from before 2015/2017 are not harmonized with those chapters, and thus, may not be considered “standardized HPTLC methods”.
Nevertheless, all TLC or HPTLC methods can be converted into standardized HPTLC methods. In a preliminary experiment, the standard and sample solutions of the original method are kept as well as developing solvent and derivatization reagent. All other parameters are set to “standard”:
- Plate material (HPTLC Silica gel 60 F254 20 x 10 cm);
- Plate layout (15 tracks, 8.0 mm bands, 8.0 mm from lower edge, 20.0 mm from left and right edges);
- Twin trough chamber, saturated for 20 minutes with saturation pad;
- Plate activation (plate conditioned at a relative humidity of 33% for 10 minutes);
- Developing distance (7.0 cm from the lower edge);
- Drying step after development (5 min with cold air);
- Derivatization (automatic spraying or dipping/immersion whenever possible);
- Digital documentation in short-wave UV light (254 nm), longwave UV light (366 nm), and white light before derivatization, long-wave UV light (366 nm), and white light after derivatization.
Other parameters can be adjusted: usually, application volumes (or concentrations of sample and standard solutions) of TLC methods are reduced to about one-fifth for HPTLC plates. Optimum application volumes for HPTLC are between 2.0 and 10.0 μL.
The preparation and use of derivatization reagents should follow the pharmacopoeias or the General SOP of the HPTLC Association. Special derivatization reagents may be evaluated.
Sample preparation may be optimized if needed and simplified if possible. Cumbersome methods and harmful solvents should be avoided. A system suitability test (SST) (e.g. based on the Universal HPTLC Mix [1]) must be introduced.
Comprehensive HPTLC fingerprinting
HPTLC is a standard technique for chemical identification of herbal drugs adopted by many pharmacopoeias. Resulting HPTLC fingerprints are usually visually evaluated. When generated with a standardized methodology, using suitable instruments and software, digital HPTLC fingerprints offer the necessary reproducibility for deeper exploitation of the data and for quantitative evaluation.
HPTLC fingerprint
The HPTLC fingerprint is the digital image of the visual HPTLC chromatogram. It represents the identity of a sample and consists of a sequence of separated zones with a certain color and intensity, and may be a stack of multiple images from different detection modes. The HPTLC fingerprint also includes any zones at the application and front positions, which are usually not detected in other chromatographic techniques.
Additionally, the HPTLC fingerprint is part of the digital image of the entire HPTLC plate. That HPTLC plate contains information regarding other samples and standards, the quality of the chromatography (assessed with an SST), and the chromatographic conditions during all steps. All information can be stored in an analysis file. All fingerprints from a plate that has passed the SST can be compared to fingerprints from other plates developed with the same method, which have also passed the SST.
Peak Profile from Image (PPI)
Unlike scanning densitometry, which measures the absorbance or fluorescence of a zone using a single wavelength per scan, image analysis evaluates the pixels of the three channels red (R), green (G), and blue (B). For each track, the RGB values of the pixel lines (RF position) of 50% of the length of the zones can be averaged and used to calculate the luminance L with the equation L = (1/3 R) + (1/3 G) + (1/3 B). Plotting the luminance as function of RF generates the Peak Profile from Image. Information on peak height and area contained in PPI data can be used for quantitative assessments.
![Figure 1: Transformation of the digital image of the HPTLC chromatogram into the corresponding peak profile from image (PPI). Adapted from [2] Figure 1: Transformation of the digital image of the HPTLC chromatogram into the corresponding peak profile from image (PPI). Adapted from [2]](/sites/default/files/styles/image_slider/public/2022-09/WP4_Fig1.png?itok=3nqdHvN6)
Figure 1: Transformation of the digital image of the HPTLC chromatogram into the corresponding peak profile from image (PPI). Adapted from [2]
The concept of “comprehensive HPTLC fingerprinting”
Conversion of images into peak profiles results in a loss of color information. Because the PPI formula weights the channels equally, the relative intensity of the zones observed in the image may differ from those of the PPI. Therefore, a comprehensive evaluation of a fingerprint includes the analysis of the PPI and the corresponding image. This is taken into account in the concept of “comprehensive HPTLC fingerprinting”.
In comprehensive HPTLC fingerprinting, tests for identity, purity, and content of an herbal drug, preparation, or product are performed in a single analysis. Qualitative and quantitative information from the HPTLC fingerprints (images in different detection modes) and PPI are combined.
Peak profiles from scanning densitometry (PPSD) can offer complementary, spectrally selective information but do not belong to the core data of comprehensive HPTLC fingerprinting.
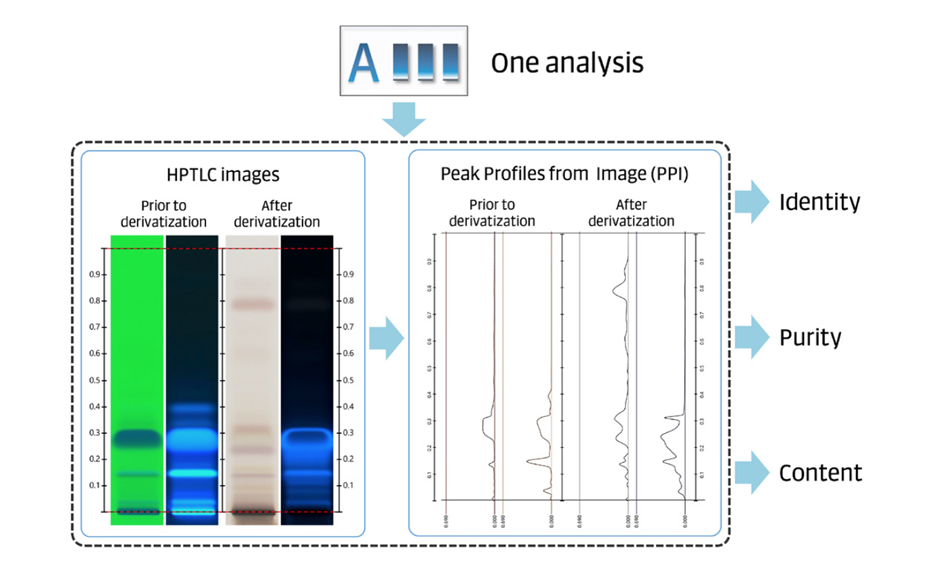
Figure 2: The combination of HPTLC images and peak profiles from images allows testing for identity, purity, and content in a single analysis.
Example: comprehensive HPTLC fingerprinting of Angelica gigas root
The concept of comprehensive HPTLC fingerprinting was developed for the quality control of the herbal drug Angelica gigas root, and can be expanded to other fields of analysis.
Criteria for the identification of A. gigas root were established based on the evaluation of multiple samples of cultivated material. An average fingerprint (pooled sample) was then generated. That fingerprint represents the typical characteristics of the herbal drug and is used as reference. The selected method is also able to distinguish A. gigas root from the roots of 27 related plant species.
Two potential confounding herbal drugs of A. gigas root, the roots of Angelica acutiloba and Angelica sinensis, which carry the same common name “Dang gui”, were investigated in a test for purity. The presence of either confounding material in a mixture is detectable at levels as low as 1%, based on the detection of z-ligustilide. This zone, characteristic of the two and nine other related herbal drugs, is absent in A. gigas root.
Literature
[1] T. K. T. Do et al. (2021) Journal of Chromatography A, 1638. DOI: 10.1016/j.chroma.2020.461830
[2] S. Cañigueral et al. Chapter 7: High performance thin-layer chromatography (HPTLC) in the quality control of herbal products. pp 119 – 136. In: Recent Advances in Pharmaceutical Sciences VIII. 2018.
![Figure 3: Identification – evaluation of multiple samples of Angelica gigas root samples, and average, representative fingerprint (track A) in short-wave UV light 254 nm (upper) and in long-wave UV light 366 nm (bottom). Adapted from [2] Figure 3: Identification – evaluation of multiple samples of Angelica gigas root samples, and average, representative fingerprint (track A) in short-wave UV light 254 nm (upper) and in long-wave UV light 366 nm (bottom). Adapted from [2]](/sites/default/files/styles/image_slider/public/2022-09/WP4_Fig3.png?itok=ME5iOXDx)
![Figure 4: Purity – detection of z-ligustilide (characteristic of the confounding species) in A. gigas root. Image and PPI evaluations. Red bars: peak height of z-ligustilide in mixtures of A. acutiloba and A. gigas root. Blue bars: peak height of z-ligustilide in mixtures of A. sinensis and A. gigas root. Adapted from [2] Figure 4: Purity – detection of z-ligustilide (characteristic of the confounding species) in A. gigas root. Image and PPI evaluations. Red bars: peak height of z-ligustilide in mixtures of A. acutiloba and A. gigas root. Blue bars: peak height of z-ligustilide in mixtures of A. sinensis and A. gigas root. Adapted from [2]](/sites/default/files/styles/image_slider/public/2022-09/WP4_Fig4.png?itok=xLdcY5wj)
![Figure 5: Test MC against a reference standard equivalent to the MC. Image and PPI evaluations, and % content of D/DA, of 24 samples of A. gigas root. Adapted from [2] Figure 5: Test MC against a reference standard equivalent to the MC. Image and PPI evaluations, and % content of D/DA, of 24 samples of A. gigas root. Adapted from [2]](/sites/default/files/styles/image_slider/public/2022-09/WP4_Fig5.png?itok=RF2YVsVx)