Dr. Daniel Jean is the founder and director of ISV (Institut des Substances Végétales, France), which produces the quantified Reference Extracts (qRE) used in this study. Dr. Ophélie Fadel is the head of the application laboratory of Chromacim (France). Dr. Debora Frommenwiler is scientist at CAMAG (Switzerland), and Dr. René De Vaumas is the CEO of Extrasynthese (France), the distributor of qRE.
Introduction
Quality control of herbal drugs, extracts and products is a challenging task because it requires proper identification and determination of content of active compounds or markers. Because monographs of the pharmacopoeias typically require independent methods for these tasks, the cost of analysis may be quite high. Reference materials are a major contribution to the cost. Quantified Reference Extracts (qRE) may be considered as suitable reference material in this context, reducing the need for stocking several separate chemical reference substances.
The goal of this work was to elaborate examples for using qRE for identification of plant materials and quantification of markers.
Standard solutions
For ginkgo, 0.2 mg/mL each of rutin and quercetin are prepared in methanol. The qRE Ginkgo biloba leaves is prepared at 30 mg/mL in methanol. For olive leaf, oleuropein is prepared at 1.0 mg/mL in methanol. For rosemary, rosmarinic acid is prepared at 0.25 mg/mL in ethanol, and the qRE Rosmarinus officinalis leaves at 1.0 mg/mL in 50% ethanol.
Sample preparation
1.0 g of powdered ginkgo leaf is mixed with 10 mL of methanol and refluxed in a water bath for 10 minutes. The mixture is centrifuged and the supernatant is used as test solution. The rosemary dried extract is prepared at 1.0 mg/mL in methanol. The qRE Olea europaea leaves (used as a sample) is prepared at 1.0 mg/mL.
Chromatogram layer
HPTLC plates silica gel 60 F254 (Merck), 20 x 10 cm are used.
Sample application
Automatic TLC Sampler (ATS 4), application as bands, 15 tracks, band length 8.0 mm, distance from left edge 20.0 mm, distance from lower edge 8.0 mm. For the ginkgo project, application volume of 3.0 μL for sample solutions and for standard solutions. For olive leave different volumes of the oleuropein standard and 10.0 μL of the qRE were applied. For rosemary, the solution of the qRE was applied with different application volumes, and the rosemary dried extract was applied with 10.0 μL.
Chromatography
In the ADC 2 with 20 min chamber saturation (with saturation pad) and after activation of the plate at 33% relative humidity for 10 min using a saturated solution of magnesium chloride. The developing distance is 70.0 mm (from the lower edge). Plates are dried for 5 min. Ethyl acetate – formic acid – acetic acid – water 100:11:11:26 (v/v) is used as developing solvent.
Post chromatographic derivatization
For rosemary and ginkgo, the plates are heated using the TLC Plate Heater (100 °C, 3 min), then derivatized with NP reagent (1.0 g 2-aminoethyl diphenylborinate in 100 mL methanol) using the Derivatizer (3.0 mL, nozzle: green, level: 4). For olive leaf, the plate is derivatized with anisaldehyde (AS) reagent (0.5 mL of p-anisaldehyde dissolved in
Documentation
TLC Visualizer in UV 254 nm, UV 366 nm, and white light prior to derivatization, and UV 366 nm, and white light after derivatization.
Densitometry
TLC Scanner 4 and visionCATS, absorbance measurement at 254 nm and 238 nm, slit dimension 5.00 mm x 0.20 mm, scanning speed 50 mm/s, for oleuropein and in fluorescence mode at 366>/400 nm for rosmarinic acid.
Results and discussion
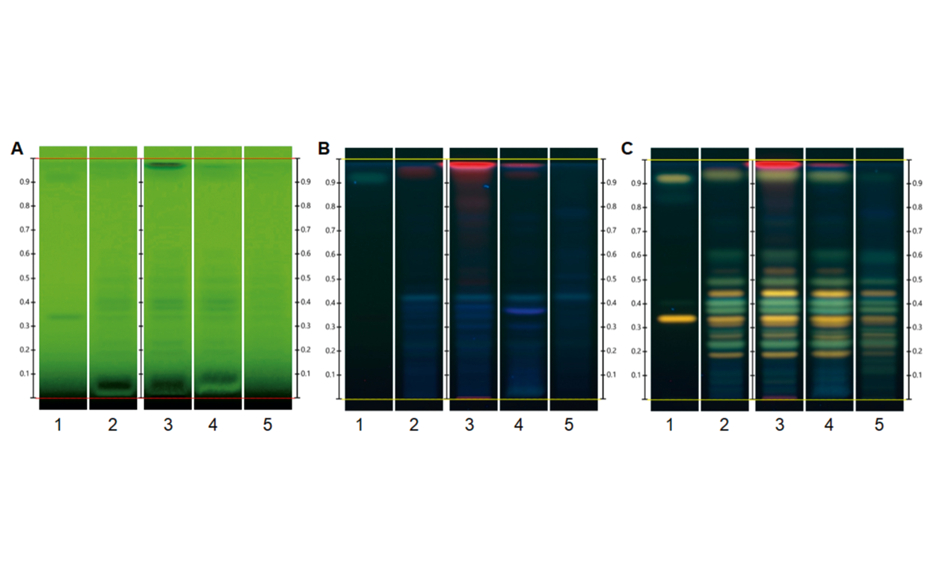
The first part of the project demonstrates the suitability of the ginkgo quantified Reference Extract as a standard for identification. The fingerprint of the qRE was compared to a reference sample of powdered ginkgo leaf extracted with methanol (track 3) or with 50% ethanol (track 4), respectively, and a market sample of ginkgo leaf dried extract (track 5). The fingerprint of the qRE resembles those of the corresponding herbal drugs and dried extract.
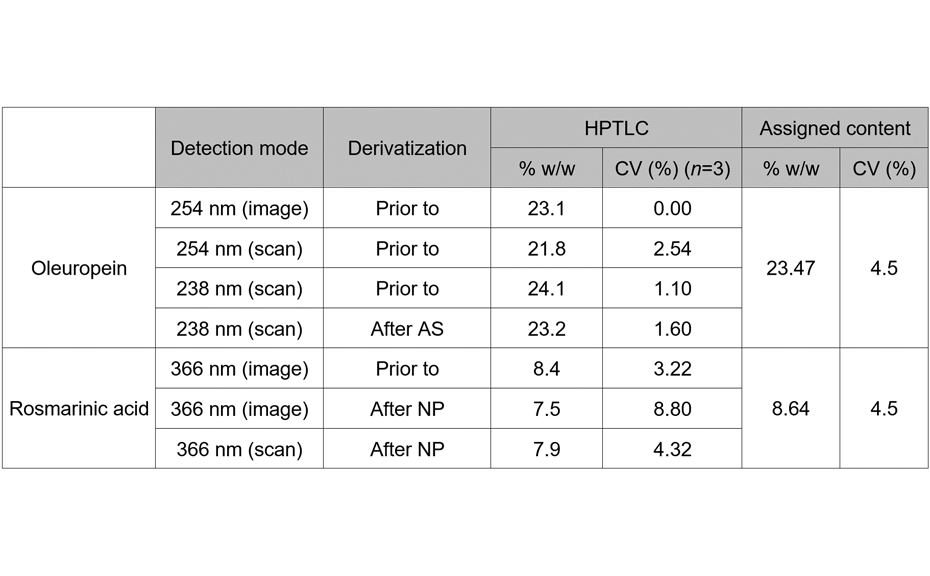
The second part of the study concerned the verification of the assigned content of oleuropein and rosmarinic acid in their respective qRE by HPTLC. Assigned contents, given in the qRE’s certificate of analysis, were initially determined by HPLC. Chemical reference substances were used for quantification, and different detection modes were compared. Comparable contents were found for both markers.
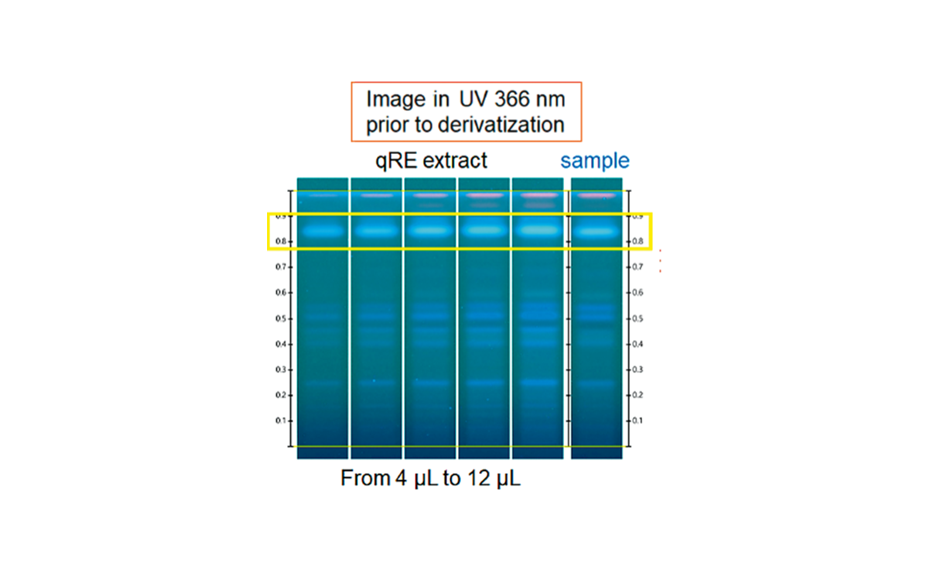
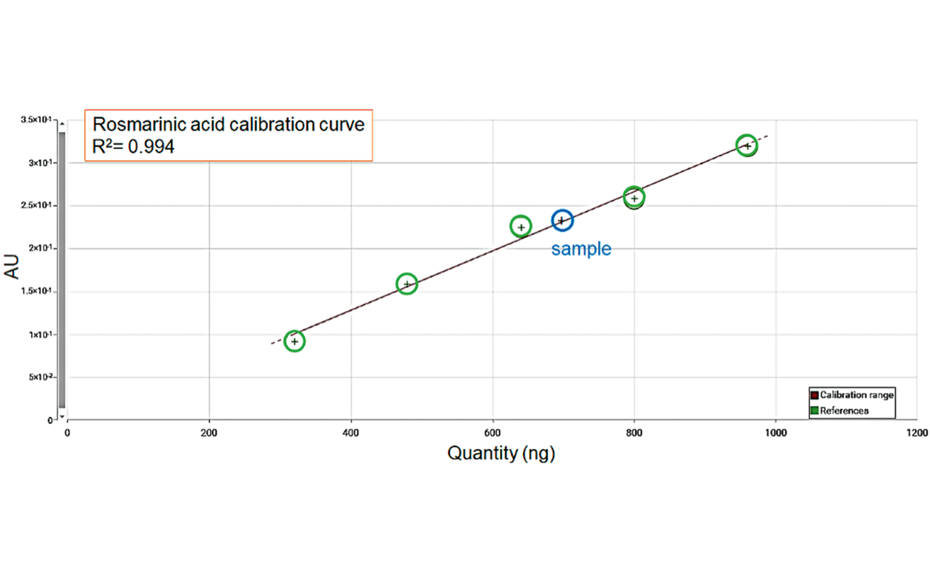
The third part of the study used one qRE and its assigned content of rosmarinic acid as reference for the quantification of rosmarinic acid in a rosemary dried extract. A solution of the qRE was applied with different application volumes to give a linear calibration curve. The content of rosmarinic acid in the sample was determined at 6.3%.
Conclusion
These examples show that qRE work well for the identification of plant materials and quantification of markers by HPTLC. For use in quality control, methods may need to be adapted from pharmacopoeia monographs.
Further information is available on request from the authors.
Contact:
Dr.Ophélie Fadel, of@chromacim.com
Dr. Debora Frommenwiler,debora.frommenwiler@camag.com
Dr. Daniel Jean, daniel.jean@insuveg.com
Dr. René De Vaumas, rdv@extrasynthese.com
Mentioned Products
The following instruments and devices were used in this work