Introduction
An SST is commonly used in routine quality control to validate the performance of an analytical system, including the method and the apparatus. In chromatography, the SST is a process that analyzes the behavior of specific reference substances under certain chromatographic conditions to know whether the method is reproducible, robust, and suitable for the intended application. In HPTLC, the SST often qualifies only a limited region of the chromatogram (e.g., specific RF values or small RF ranges due to the need for having barely separable substances). If no deviation from the acceptance criteria is observed, the entire chromatographic system is considered compliant. However, in practice, the chromatographic quality of the other regions remains unknown. Additionally, HPTLC methods using developing solvents of different polarity and selectivity may require different sets of substances for SST. Some substances are costly and not readily available, which can increase the cost of analysis. To overcome these problems, a Universal HPTLC Mix (UHM) for use in SST was developed [1].
With the UHM, HPTLC laboratories have a single solution, applicable as SST to a wide range of chromatographic systems, with different polarities and selectivities. Its low price, stability in solution, and capability to detect small chromatographic variations make the UHM particularly attractive. The replacement of conventional substances for SST by the UHM will help laboratories to save time and money required for laborious investigation of specific reference substances for each method to be qualified. Different fields of application can benefit from the UHM concept, such as herbal drugs, forensics, pharmaceuticals, cosmetics, etc.
Standard solutions
Sulisobenzone, thymidine, paracetamol, 9-hydroxyfluorene and 2-(2H-benzotriazol-2-yl)-4-(1,1,3,3-tetramethylbutyl)phenol were prepared at 1 mg/mL. Guanosine is prepared at 0.5 mg/mL, thioxanthen-9-one at 0.01 mg/mL, and phthalimide at 2 mg/mL. All substances are dissolved in methanol.
Chromatogram layer
HPTLC plates silica gel 60 F254 (Merck), 20 x10 cm are used.
Sample application
2.0 μL of solutions are applied as bands with the Automatic TLC Sampler (ATS 4), 15 tracks, band length 8.0 mm, distance from left edge 20.0 mm, distance from lower edge 8.0 mm.
Chromatography
Plates are developed to 70mm(fromthe lower edge) in the ADC 2 with chamber saturation (20 min, with filter paper) and after activation at 33% relative humidity for 10 min using a saturated aqueous solution of magnesium chloride. 20 different developing solvents (eight of them are listed below) were investigated, followed by drying for 5 min.
Documentation
Images of the plate are captured with the TLC Visualizer 2 at UV 254 nm and 366 nm.
Densitometry
Absorbance measurement at 254 nm and fluorescence measurement at 366 nm with TLC Scanner 4 and visionCATS, slit dimension 5.00 mm x 0.20 mm, scanning speed 20 mm/s. For the fluorescence measurement, a mercury lamp and a cut-off filter at 400 nm are used.
Results and discussion
In the first step of the investigation, the suitable substances for the UHM were selected. The researchers [1] considered the following criteria, which lead to a list of 56 candidates: 1) Low hazard (not harmful and non-toxic substances); 2) Detectability at UV 254 and 366 nm prior to derivatization; 3) Stability in solution for at least two months; 4) Low cost (<50 CHF/g).
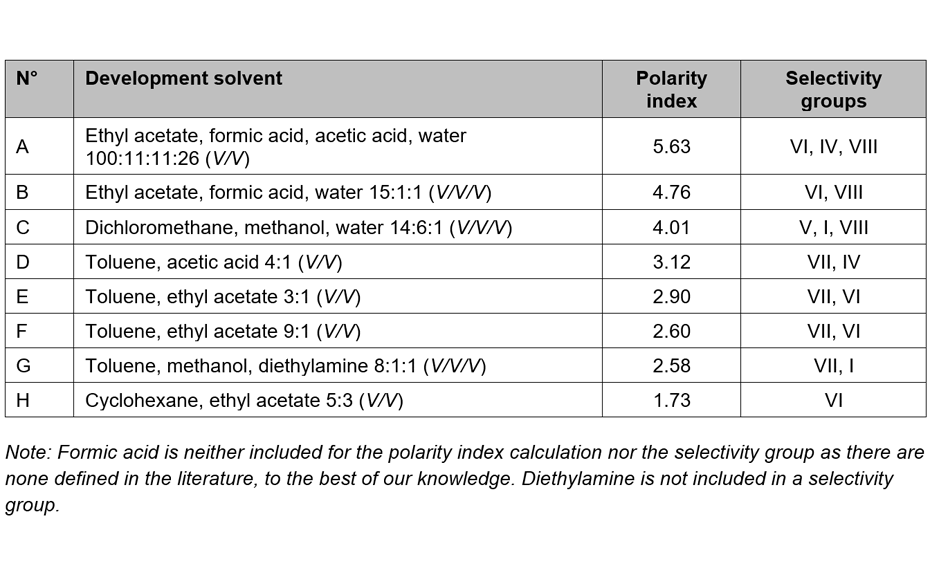
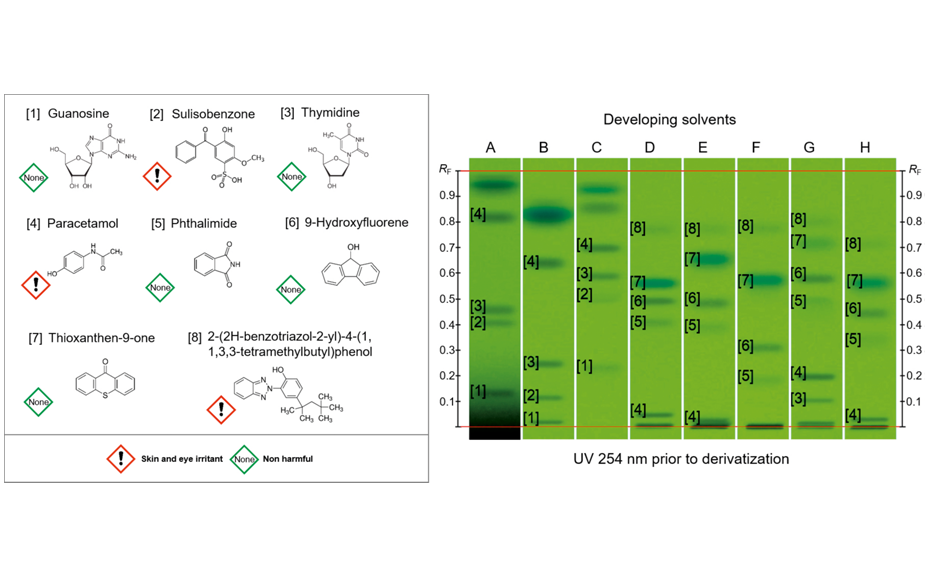
The chromatographic behavior of those 56 standards was evaluated with 20 developing solvents, covering a wide range of polarity and selectivity. The objective was to find a smaller group of substances that achieves an even distribution throughout the entire chromatogram for the maximum number of different developing solvents. Additionally, each developing solvent should achieve baseline separation for at least 3 – 4 substances. The chosen substances and their fingerprints in eight different developing solvents are shown in the following image.
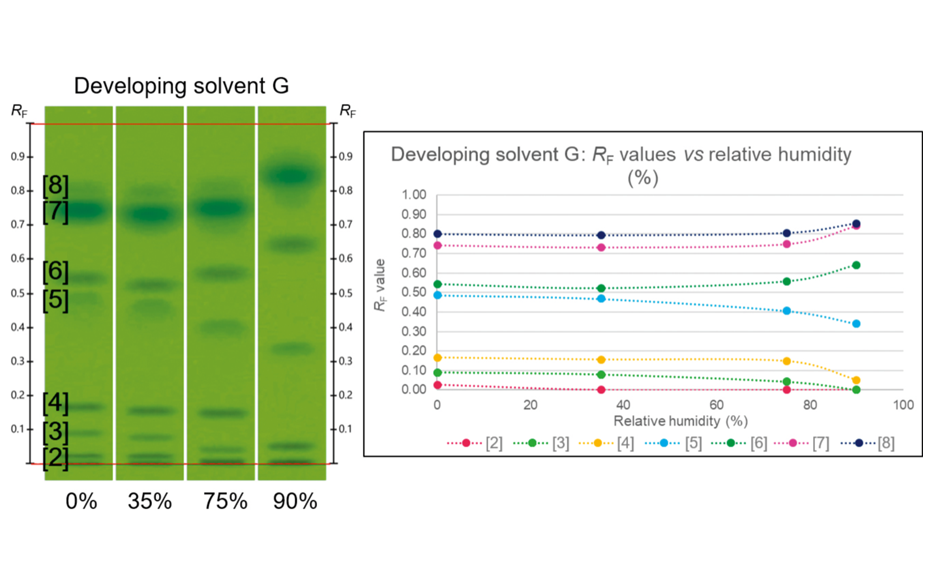
To evaluate, whether the UHM responds to variations of the chromatographic conditions, three experiments were performed. In the first, plates were conditioned to different relative humidity (from 0% to 90%) prior to development. As shown in the image below, the UHM is sensitive to variations in relative humidity, particularly to the higher ones. The differences are more expressive if the developing solvent contains no water.
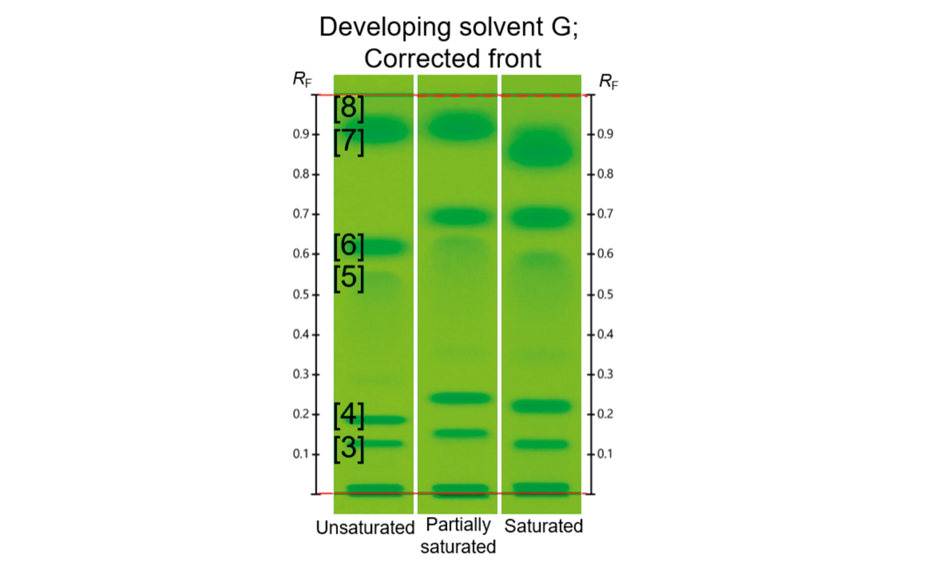
In the second experiment, the individual proportion of the solvents in developing solvents B and F was changed (±10%), and the effect on the chromatography was evaluated. A difference of up to 0.06 RF units was observed from the mean RF values of the control track. In the third experiment, different levels of chamber saturation were tested: unsaturated, partially saturated (20 min, no filter paper), and saturated (20 min, with filter paper). RF values increased with partial saturation, but then decreased with full saturation, proving that the UHM can detect chamber saturation problems.
The UHM performance was evaluated in intra- and inter-laboratory tests based on the ΔRF in developing solvents B, F and G. For the intra-laboratory test, the confidence interval ΔRF was 0.03, while for the inter-laboratory test, this value was 0.04.
[1] T. K. T. Do, M. Schmid, M. Phanse, A. Charegaonkar, H. Sprecher, M. Obkircher, E. Reich. Development of the first universal mixture for use in system suitability tests for High- Performance Thin-Layer Chromatography. J Chromatogr A, 1638 (2021). DOI: 10.1016/j.chroma.2020.461830.
Further information is available on request from the authors.
Contact: Dr. Tiên Do, CAMAG Laboratory, Sonnenmattstrasse 11, 4132 Muttenz, Switzerland, tien.do@camag.com
For more information about the Universal HPTLC Mix (UHM), please visit the Sigma-Aldrich website.
Mentioned Products
The following instruments and devices were used in this work