Introduction
Chlorinated paraffins (CP) are complex mixtures of polychlorinated n-alkanes with 10 to 30 carbon atoms and variable chlorine contents (30–70%). CP are categorized by their carbon chain length as short- (SCCP; C10–C13), medium- (MCCP; C14–C17), and long-chain (LCCP; C18–C30) CP and they are widely used for technical applications, e.g., they are added to plasticizers, paints, and flame retardants. CP are known to be persistent, and their bioaccumulation depends on the carbon chain length and the degree of chlorination. Because of their high toxicity, SCCP were classified as persistent organic pollutants (POP) according to the Stockholm Convention in 2017. MCCP and LCCP have similar chemical properties as SCCP, however, there have been only few studies about their toxicity and bioaccumulation, and their metabolism and distribution in the environment up to now, why they also pose a very high risk to humans. Due to their chemical structure, the degradation of CP in the environment is negligible, while accumulation in lipophilic tissues is very likely and high quantities might occur in lipids. CP were already detected in vegetable oils, milk, and dairy products, as well as in human milk fat and additionally in food supplements with high fat content. The analysis of CP is a very challenging task because CP are complex mixtures of polychlorinated n-alkanes, comprising thousands of congeners. The separation of these complex mixtures of unknown isomers and congeners is even not possible by gas chromatography (GC). Therefore, the presented screening method for CP uses the pSPE concept that quantifies CP as the sum. Based on the cost-effective and efficient HPTLC technique, pSPE guarantees reliable results for many samples simultaneously in a short time [1].
The determination of CP by applying the pSPE concept was successfully developed for a rapid and selective screening of CP as the sum. After sulfuric acid treatment and liquid-liquid partition into n-hexane, pSPE was performed on silica gel plates employing a twofold development. The analytes, which were focused in a single target zone, were determined by a densitometric absorption scan after derivatization with o-tolidine showing the total CP content, and the amounts were calculated as the sum by means of a reference CP. A comparison of the results obtained for the total CP in dietary supplements by pSPE with visual detection (pSPE–VIS) and by GC with highresolution mass spectrometry (GC–HRMS) proved the pSPE approach as reliable tool for screening purposes.
Standard solutions
For method development, technical CP mixtures (SCCP with chlorine contents of 51.5%, 55.5%, and 63% and MCCP with chlorine contents of 42%, 52%, and 57%) are diluted in n-hexane to a concentration of 25 ng/μL. For the determination of the limit of decision and quantitation, recovery experiments in vegetable oils, and the analysis of dietary supplements, the reference CP (MCCP with a chlorine content of 52%) is diluted to concentrations of 0.075–0.8 ng/μL, 0.75–10 ng/μL, and 0.25–10 ng/μL, respectively. The internal standard (ISTD) 4,4-DDT is prepared at a concentration of 400 μg/mL (stock solution) and used at a final concentration of 6 ng/μL in all samples and standards. [1] For GC–HRMS measurements, SCCP calibration standards with chlorine contents of 51.5%, 53.5%, 55.5%, 59.25%, and 63% and MCCP standards with chlorine contents of 42%, 47%, 52%, 54.5%, and 57% are prepared by respective dilutions of technical CP mixtures to a concentration of 10 ng/μL. The ISTD α-PDHCH and ε-HCH are added at concentrations of 0.075 and 0.05 ng/μL to all samples and standards. [1]
Sample preparation
Vegetable oil is treated according to Coelhan [2] with somemodifications. After the addition of 15 μL of the ISTD 4,4-DDT stock solution to 250 mg of vegetable oil, 500 μL of n-hexane and 2.5 mL of concentrated sulfuric acid are added and the mixture is shaken for 3 min at 2200 rpm prior to centrifugation for 30 min. The n-hexane phase is separated, and the extraction is repeated with 500 μL of n-hexane under the same conditions. Both n-hexane extracts are combined and used for pSPE [1]. Oil-based dietary supplement capsules (250 mg of oil) are extracted by the same procedure as described above for the vegetable oil (following the procedure of the sulfuric acid treatment of the sample solution in n-hexane) including the addition of 7.5 μL of the ISTD α-PDHCH prior to the extraction (for GC–HRMS analysis). Furthermore, the combined n-hexane extracts (≈1 mL) are shaken with 2 mL of sulfuric acid for 4 min prior to centrifugation for 5 min. After separation of the n-hexane phase, the sulfuric acid treatment is repeated under the same conditions. A 300 μL aliquot of the n-hexane extract is used for pSPE and a 400 μL aliquot of the n-hexane extract including 2 μL of the ISTD ε-HCH is used for GC–HRMS. [1]
Chromatogram layer
HPTLC plates silica gel 60 (Merck), 20 x 10 cm are used.
Sample application
30 μL of samples and standards are applied as areas of 6 mm x 3 mm with the Automatic TLC Sampler (ATS 4), 22 tracks, distance from the left edge 13.0 mm and from the lower edge 8.0 mm. After application, plates are dried in a fume hood for 5 min.
Chromatography
Plates are developed twofold in the ADC 2 after activation at 33% relative humidity for 5 min using a saturated solution of magnesium chloride. The 1st development is performed with cyclohexane – toluene 94:6 (V/V) to a migration distance of 80 mm and the 2nd development is done with dichloromethane – n-hexane 90:10 (V/V) to a migration distance of 50 mm followed by a drying time of 3 min after each development.
Post-chromatographic derivatization
The plate is immersed into a solution of o-tolidine (4% in acetone) using the Chromatogram Immersion Device with an immersion speed of 3 cm/s and an immersion time of 1 s, dried for 4 min in a stream of cold air, and irradiated with UV-C light inside a self-made irradiation device using a cycle with irradiation and cooling steps [1].
Documentation
Images of the plate are documented with the TLC Visualizer in white light.
Densitometry
Absorbance measurement at 645 nm is performed with the TLC Scanner 4 with a scanning speed of 20 mm/s, a data resolution of 100 μm/step, and a slit dimension of 5 mm x 0.30 mm using the manual detector mode applying a quick scan range of 47–53 mm on the track of the most concentrated standard. For quantitation, the respective peak areas are used.
Gas chromatography coupled to high-resolution mass spectrometry
GC–HRMS measurements with electron capture negative ionization (ECNI) are performed according to the method of Krätschmer et al. [3] and quantitation is done according to Sprengel et al. [4] and Reth et al. [5].
Results and discussion
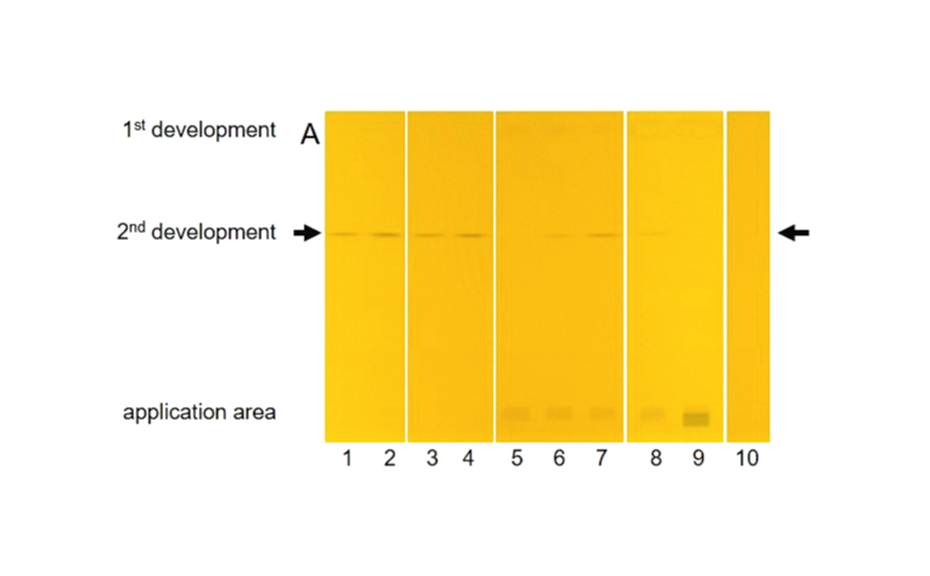
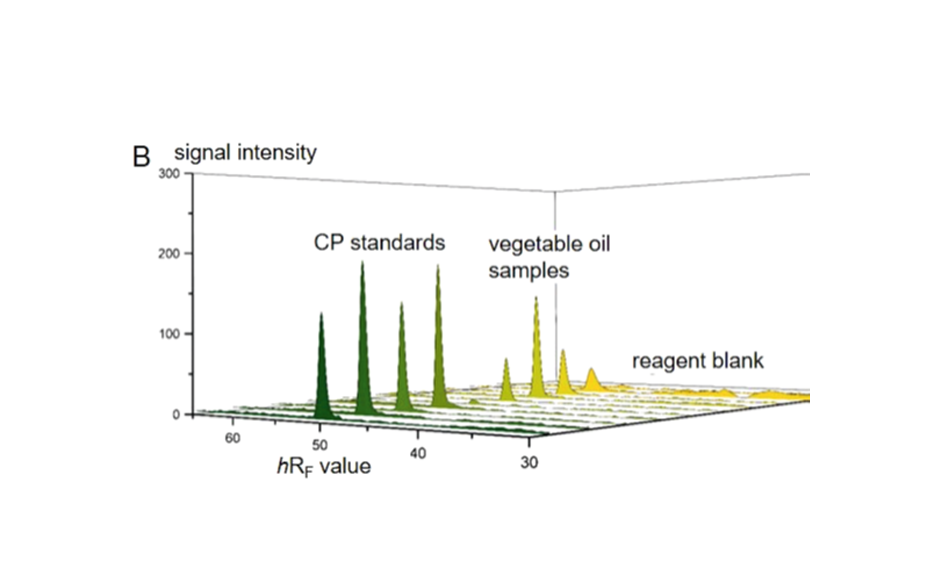
The determination of CP as the sum was performed by the straightforward pSPE approach. The target analytes were selectively separated from the matrix and additionally focused in a single and sharp target zone since all representatives of the chemical group of CP have almost identical properties in regard to a chromatographic separation. Best results were obtained by a twofold development on HPTLC silica gel with cyclohexane – toluene 94:6 (V/V) for the 1st development and dichloromethane – n-hexane 90:10 (V/V) for the 2nd development. In the target zone, the selective determination of the total CP as the sum was possible directly on the plate after derivatization with o-tolidine and UV irradiation by means of an absorption scan at 645 nm. For quantitation, a reference CP (MCCP with a chlorine content of 52%) was used.
Limits of decision and quantitation for the reference CP were determined according to the DIN 32645 calibration method [6] in the range of 2.25–24 ng CP/zone (corresponding to 0.3–3.2 mg CP/kg of vegetable oil) and 1.5–4.9 ng CP/zone (corresponding to 0.2–0.7 mg CP/kg of vegetable oil), respectively, with relative standard deviations (RSD) <5%. Thus, the presented pSPE–VIS approach delivers a suitable screening tool for the analysis of the total CP down to ~1 mg/kg of vegetable oil, while for lower limits, sample application volume can be increased.
Recoveries for CP in sunflower oil, olive oil, and rice bran oil at levels of 5, 10, and 30 mg reference CP/kg were very close to 100% after the subtraction of a slight impurity originating from the sample preparation. With precision of recovery expressed as RSD <4%, the method was very well repeatable for all tested vegetable oils and spiking levels.
Comparison of results obtained by pSPE–VIS and GC–HRMS for six dietary supplement samples (oil-based food supplements) showed generally conformity. The total CP contents determined by pSPE–VIS were in the same order of magnitude compared to the contents analyzed by GC–HRMS and results were well repeatable. Only one sample (sample 3) showed a clearly higher content by pSPE, however, displayed a different color of the target zone that evidently originated from comigrated matrix components. In general, large deviations in results are well known for the very challenging CP analysis, which depend on the used GC–MS set-up including the calibration standards and the calibration method.
In summary, the pSPE screening approach for the determination of the total CP provides a very useful alternative to the time-consuming and very complicated GC approaches that analyze the individual components and calculate the sum afterwards. Furthermore, in terms of a rapid screening, pSPE offers the chance to save time and costs, because only the suspected and questionable samples need additionally to be analyzed by GC/ECNI–HRMS.
[1] C. Oellig et al., J Chromatogr A (2019) 460380.
[2] M. Coelhan, Anal Chem (1999) 4498.
[3] K. Krätschmer et al., J Chromatogr A (2018) 53.
[4] J. Sprengel et al., Rapid Commun Mass Spectrom (2018) 49.
[5] M. Reth et al., J Chromatogr A (2005) 225.
[6] Deutsches Institut für Normung, DIN 32645, Beuth, Berlin, 2008.
Further information is available on request from the authors.
Contact: PD Dr. habil. Claudia Oellig, Department of Food Chemistry and Analytical Chemistry (170a), Institute of Food Chemistry, University of Hohenheim, 70599 Stuttgart, claudia.oellig@uni-hohenheim.de
Mentioned Products
The following instruments and devices were used in this work (discontinued products are replaced with current versions)
![Mean recoveries of CP from vegetable oils in % ± standard deviation (n = 4) at spiking levels of 5, 10, and 30 mg reference CP/kg of oil. Modified from [1]. Mean recoveries of CP from vegetable oils in % ± standard deviation (n = 4) at spiking levels of 5, 10, and 30 mg reference CP/kg of oil. Modified from [1].](/sites/default/files/styles/image_slider/public/2022-04/CBS%20128_Screening%20for%20chlorinated%20paraffins_tbl1.png?itok=tIyBa7kG)
![Total CP amounts in six dietary supplement samples from the European market by pSPE–VIS and GC/ECNI–HRMS in mg reference CP/kg of sample ± standard deviation (n = 4). Modified from [1]. Total CP amounts in six dietary supplement samples from the European market by pSPE–VIS and GC/ECNI–HRMS in mg reference CP/kg of sample ± standard deviation (n = 4). Modified from [1].](/sites/default/files/styles/image_slider/public/2022-04/CBS%20128_Screening%20for%20chlorinated%20paraffins_tbl2.png?itok=erIfvmbp)



