Dr. Kashyap Thummar, Assistant Professor at Graduate School of Pharmacy (GSP), Gujarat Technological University (GTU), India, employs chromatographic separation techniques, especially HPTLC, to develop new and improved quantitative analytical methods for determination of drugs, impurities, adulteration and naturally occurring compounds in a variety of sample matrices. He prefers HPTLC because it is flexible, inexpensive, time-saving and does not produce toxic waste.
Introduction
Phthalates are esters of phthalic acid that are commonly added to plastics to improve their flexibility, transparency, durability, and longevity. These phthalates are easily released into the environment from plastic and can harm all living organisms. Phthalates enter the body by contact to plastics, e.g. through ingestion, inhalation, skin absorption, and intravenous injection.
For the detection of extractable and leachable phthalates in pharmaceutical products, a simple, rapid, precise, and accurate HPTLC method was developed. It simultaneously estimates the presence of four different phthalates in various pharmaceutical products and containers: dimethyl phthalate (DMP), diethyl phthalate (DEP), dibutyl phthalate (DBP) and di(2-ethylhexyl) phthalate (DEHP). The method was successfully applied for determination of extractables and leachables from 12 parenteral products packed in plastic containers.
Standard solutions
10.0 mg of each phthalate were individually dissolved in 10.0 mL of methanol and subsequently combined to prepare a working standard solution at a concentration of 0.1 mg/mL of each.
Sample preparation
2.0 g of the plastic container (extractable) and 30.0 mL of the product packed in it (leachable) are extracted 3 times with 30.0 mL of n-hexane by 10 min of sonication. The organic layers are collected, evaporate to dryness and reconstituted with 1.0 mL of methanol.
Chromatogram layer
HPTLC plates silica gel 60 F254 (Merck), 20 x 10 cm are used.
Sample application
Samples and standard solutions are applied as bands with the Linomat 5, 20 tracks, band length 5.0 mm, distance from left edge 10.0 mm, distance from lower edge 8.0 mm. 20.0 μL for sample solutions and 1.0–14.0 μL for standard solutions (8 points for calibration) are applied.
Chromatography
Plates are developed in a saturated Twin Trough Chamber (15 min, with filter paper) with n-hexane – ethyl acetate 9:1 (V/V) to a migration distance of 90 mm (from the lower edge), followed by drying with cool air for 5 min.
Documentation
Images of the plate are captured with the TLC Visualizer in UV 254 nm.
Densitometry
Absorbance measurement at 240 nm is performed with the TLC Scanner 4 and winCATS, slit dimension 4.00 mm x 0.30 mm, scanning speed 20 mm/s.
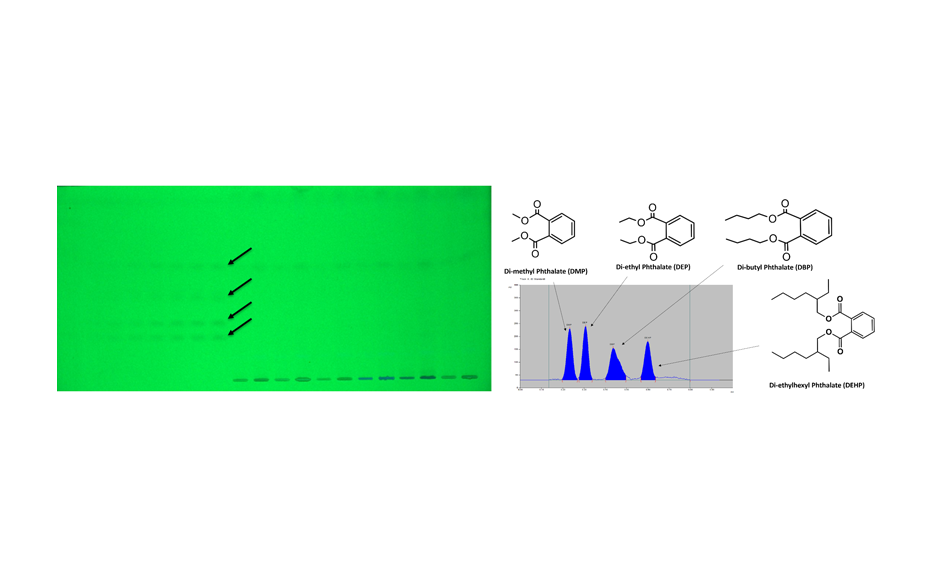
HPTLC chromatogram in UV 254 nm (left) and densitogram measured at 240 nm (right)
Results and discussion
A representative densitogram of samples is shown. During evaluation of the chromatogram, phthalates in simulated and test samples give the same RF values as the standard and are well separated from matrix components. A simulated sample is a laboratory-made sample that is designed to mimic a real-world product or material.
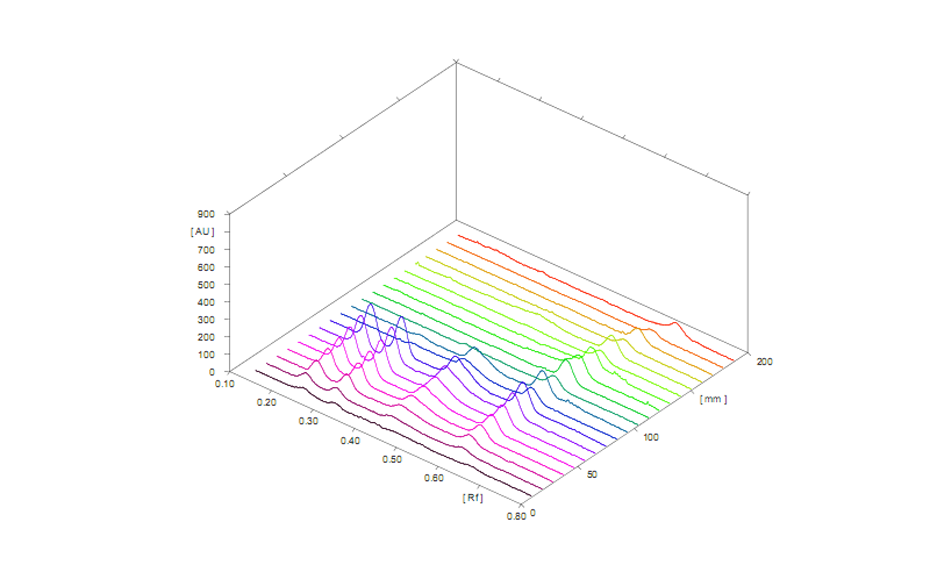
3D profile of scanned sample and standard tracks (measured at 240 nm)
DMP, DEP, DBP and DEHP are separated at RF values of 0.23, 0.31, 0.44 and 0.60 respectively. The limit of quantification was in the range of 41.7 to 99.8 ng/band for the four phthalates and linearity was established between 100.0 and 1400.0 ng/band. The presence of individual phthalates was found in 12 pharmaceutical products (all parenteral formulations) in significant amounts. Importantly, of the four phthalates, DEHP was found in all tested samples as an extractable and leachable. The HPTLC method for detection of phthalates is cost-effective as compared to available analytical methods such as HPLC, LCMS/ MS, GCMS/MS etc. as these techniques require more solvent consumption, power consumption, analysis time, and involve complex sample preparation methods. The method can be universally applied to other samples apart from pharmaceutical products like water, food items etc. which are sold in plastic containers.
[1] T. H. Broschard et al. (2016) Regulatory Toxicology and Pharmacology 81, 201–211.
[2] K. Thummar and N. Sheth, Publication date: 2022/6/7, Patent office: IN, Patent number: 398670.
[3] K. Thummar et al. (2020) Analytical Chemistry Letters 10 (1), 93–103.
Further information is available on request from the authors.
Contact: Dr. Kashyap Thummar, Assistant Professor, Graduate School of Pharmacy, Gujarat Technological University, Gandhinagar, Gujarat, India, ap_kashya@gtu.edu.in