Dr. Wilmer H. Perera is the Lab Manager at CAMAG Scientific, Inc. in Wilmington, NC and he is dedicated to the development of HPTLC methodologies that can be applied to the dietary supplement, food and cosmetic industries, and more to come. Dr. Michael Lelah and Mallory Goggans are with NutriScience Innovations, a dietary ingredient development and distribution company with headquarters in Milford, CT. Dr. David Bom is a consultant for NutriScience. NutriScience is the developer of ButyraGen™.
Introduction
ButyraGen™ is a new dietary ingredient, a prebiotic direct butyrate generator [1]. The primary active ingredient, tributyrin (glycerol with three butyrate arms), is hydrolyzed in the body to the short chain fatty acid butyrate (butyric acid). Butyrate is a postbiotic involved in supporting digestive health through reducing gut permeability and also is an important gut signaling molecule for the gut-brain axis and other organ support [2]. Although tributyrin itself is an oil, ButyraGen™ is a spray-dried powder. This makes ButyraGen™ a hybrid – the material is a powder but it contains an oil. Dietary ingredients for use in dietary supplements manufactured under cGMP, require testing for identity, purity, strength and composition [3]. The identity test can also be used as a test for adulteration, which is a general concern for dietary ingredients and supplements. The identity test can help confirm whether an ingredient has been adulterated. HPTLC is widely used in the dietary supplement industry for the identification of botanicals, botanical concentrates and botanical extracts. The purpose of this study was to develop an HPTLC method for the identification of ButyraGen™ using the identification of tributyrin, the main active ingredient in ButyraGen™ (> 50% content) as the primary identification marker. The suitability of the method for this purpose was determined using tributyrin as a standard and also by comparing it against other fatty acids and lipids. Suitability is fit for purpose, which is the appropriate standard for the development of an identification method for a dietary ingredient [4]. Commonly used and inexpensive food fatty acids and oils are compared to determine if the method is sufficiently sensitive and specific to distinguish ButyraGen™ and tributyrin from these materials, which may be considered potential adulterants. Additionally, a negative control consisting of the other components of ButyraGen™ (without tributyrin) was evaluated to determine the effect of these other components in the product.
The use of HPTLC for the identification of oils is far less well known although methods for the determination of fatty oils have been developed [5]. Many manufacturers of dietary ingredients and dietary supplements have HPTLC instrumentation in their analytical labs and conduct identity testing of botanicals on a regular basis. Thus, HPTLC is an idealmethod to identify tributyrin in ButyraGen™but it can be used for many other applications. The HPTLC PRO System boosts the applicability of the technique since it is a fully automated system where multiple samples can be analyzed in sequence, overcoming the environmental effects produced by the previous open system. HPTLC PRO also adds a more rigorous control of the gas phase and although still under development as an analytical tool, it will become a standard and powerful technique for advanced research and quality control purposes.
Standard solutions
4.0 mg of tributyrin and triacetin are dissolved in 1.0 mL ofmethanol. The Universal HPTLC Mix (UHM) solution was prepared as described in literature [6] and used as system suitability test (SST).
Sample preparation
ButyraGen™and ButyraGen™placebo (ButyraGen™ without the primary active tributyrin), glycerol monostearate, raw cocoa butter and palm kernel oil were prepared at 10.0 mg/mL methanol. 20.0 μL of medium chain triglycerides and linseed oil were dissolved in 980.0 μL of methanol and toluene, respectively. Samples were sonicated for 10 min at room temperature and centrifuged at 3000 rpm for 5 min as needed. The supernatant was used for further analysis.
Chromatogram layer
HPTLC plates silica gel 60 F254 (Merck), 20 x 10 cm were used.
Sample application
10.0 μL of ButyraGen™and ButyraGen™placebo, glycerol monostearate, raw cocoa butter solutions, 2.0 μL of medium chain triglycerides, palm kernel oil and linseed oil solution while 40.0 μL and 20.0 μL of triacetin and tributyrin solutions, respectively, are applied as bands with the HPTLC PRO Module APPLICATION, 15 tracks, band length 8.0 mm, distance from the left edge 20.0 mm, track distance 11.4 mm, distance from the lower edge 8.0 mm. The first rinsing step (bottle 1 solvent) is done with methanol – acetonitrile – iso-propanol – water – formic acid 250:250:250:250:1 (V/V) and the second rinsing step (bottle 2 solvent) is done with methanol – water 7:3 (V/V).
Chromatography
Chromatography Plates were developed with the three developing solvents in the ADC 2 with activation of the plate at 33% relative humidity for 10 min using a saturated solution of magnesium chloride. LPDS (toluene, ethyl acetate 9:1 (V/V)) is used without saturation, whereas MPDS (cyclopentyl methyl ether, tetrahydrofuran, water, formic acid 40:24:1:1 V/V)) and HPDS (ethanol, dichloromethane, water, formic acid 16:16:4:1 (V/V)) are used with 20 min chamber saturation (with saturation pad). The developing distance for all three methods was 70 mm (from the lower edge). Plates were dried for 5 min.
Post-chromatographic derivatization
The plate was immersed into primuline (0.05% in acetone – water, 4:1 (V/V)) using the Chromatogram Immersion Device 3, immersion speed 3 cm/s and immersion time 5 s, dried for 5min with cold air.
Documentation
Images of the plate are captured with the TLC Visualizer 2 in UV 254 nm prior to derivatization and in UV 366 nm after derivatization.
Results and discussion
The HPTLC analysis was qualified by using an UHM as system suitability test [6]. Three main quenching zones were observed in short wavelength UV 254 nm for the SST with RF 0.11 ± 0.04, 0.21 ± 0.04 and 0.76 ± 0.04 in the figure below. The results are quite straightforward, ButyraGen™ (track 4) is identified by the tributyrin reference standard (track 3) and none of the other fatty acids tested should moved to this position. The other materials tested represent a range of food and other fatty acids which potentially could be used as adulterants to replace tributyrin in ButyraGen™. Tributyrin is a triglyceride with a glycerol backbone and three butyrate side chains. Triacetin is a triglyceride with a glycerol backbone and three acetate side chains. Glycerol monostearate is a long chain monoglyceride commonly used as a food emulsifier. The main constituent in cocoa butter is the triglyceride derived from palmitic, oleic and stearic acid. Cocoa butter also contains other unsaturated and saturated fatty acids. Medium chain triglycerides are triglycerides with two or three medium chain fatty acids. Palm kernel oil is high in saturated fats and lauric acid. Linseed oil (also known as flax seed oil) is high in unsaturated diglycerides and triglycerides, including alpha-linoleic acid.
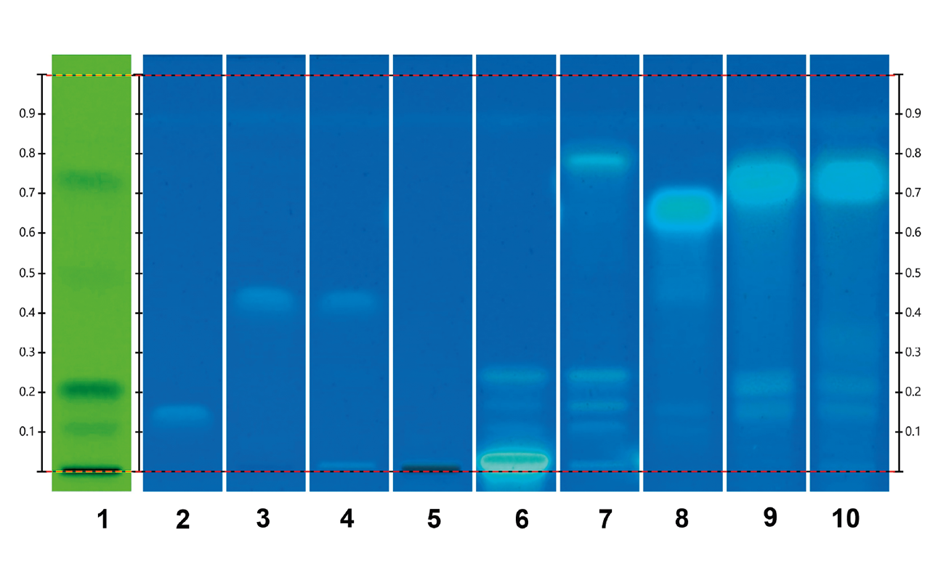
HPTLC analysis of the UHM (track 1) in UV 254, triacetin and tributyrin (tracks 2 and 3), ButyraGen™ and ButyraGen™ placebo (tracks 4 and 5), glycerol monosterate, raw cocoa butter, medium chain triglycerides, palm kernel oil and linseed oil (tracks 6–10) in UV 366 nm post derivatization with primuline solution.
This method of HPTLC chromatographic separation is very specific for the different types of mono-, di-, and triglycerides indicating very good specificity for tributyrin and ButyraGen™. These results indicate the suitability (fit for purpose) of the method for the identification of tributyrin and ButyraGen™. Certainly, for the wide range of pure and naturally occurring complex fatty acid esters tested here, ButyraGen™ and tributyrin are completely and specifically distinguished. In the event that ButyraGen™ was to be adulterated with any of these products, this identity test method will be able to confirm the presence of such an adulterant. This indicates the method as suitable for confirming the presence of a variety of potential adulterants.
[1] https://nutriscienceusa.com/product/butyragen
[2] Canani R.B. et al. World J Gastroenterol 17(12) (2011) 1519-1528.
[3] FDA, Code of Federal Regulations, 21CFR111.70. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/ cfcfr/CFRSearch.cfm?fr=111.70&SearchTerm=identity
[4] Wenclawiak B. et al. Quality Assurance in Analytical Chemistry (2010) 215-245.
[5] Identification of fixed oils, HPTLC Association https://www.hptlc-association.org/methods/methods_ for_identification_of_herbals.cfm
[6] Do T.K.T. et al. J Chromatogr A 1638 (2021) 461830.
Further information is available on request from the authors.
Contact: Dr. Wilmer H. Perera, CAMAG Scientific, Inc., 515 Cornelius Harnett Drive Wilmington, NC 28401, USA, wilmer.perera@camag.com
Dr. Michael Lelah, NutriScience Innovations, 130 Old Gate Lane, Unit C, Milford, CT 06460, mlelah@nutriscienceusa.com